Журнал «Медицина неотложных состояний» Том 21, №5, 2025
Вернуться к номеру
Кальцій-спрямована терапія при гострому панкреатиті
Авторы: Чуклін С.М., Чуклін С.С.
Медичний центр Святої Параскеви, м. Львів, Україна
Рубрики: Медицина неотложных состояний
Разделы: Справочник специалиста
Версия для печати
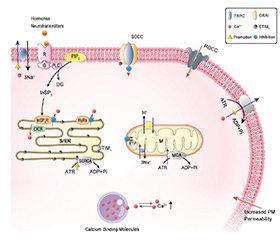
Актуальність. Гострий панкреатит (ГП) є поширеним і потенційно небезпечним для життя захворюванням, яке супроводжується високим рівнем ускладнень і летальності. Одним із ключових механізмів його патогенезу є порушення кальцієвого гомеостазу в ацинарних клітинах підшлункової залози. Надмірне накопичення іонів кальцію в цитозолі запускає передчасну активацію ферментів, мітохондріальну дисфункцію, оксидативний стрес і системну запальну відповідь. У зв’язку з цим розробка терапевтичних стратегій, спрямованих на нормалізацію внутрішньоклітинного кальцієвого балансу, є перспективним напрямком патогенетичного лікування. Мета: проаналізувати сучасні експериментальні й клінічні дослідження, що стосуються ролі кальцієвого дисбалансу в патогенезі гострого панкреатиту, та оцінити ефективність різних напрямів його фармакологічної корекції. Матеріали та методи. Проведено огляд літератури з баз даних PubMed, Scopus і Google Scholar до березня 2025 року. До аналізу включено експериментальні (in vitro, in vivo) та клінічні дослідження, присвячені механізмам порушення кальцієвого обміну при ГП і впливу різних фармакологічних агентів на його корекцію. Результати. Розглянуто основні молекулярні мішені кальцій-спрямованої терапії, зокрема IP3- і ріанодинові рецептори, SOC/CRAC-канали, TMEM16A, сигнальні шляхи PI3K/Akt і кальциневрин/NFAT. Наведено дані щодо ефективності таких засобів, як кофеїн, дантролен, докозагексаєнова кислота (DHA), інгібітори каналів Orai1 (CM4620/Auxora, GSK-7975A), TMEM16A-інгібітори, інсулін, хелатори кальцію (BAPTA-AM), інгібітори кальциневрину (циклоспорин A, такролімус) і мікроРНК (miR-26a). Ці сполуки впливають на зменшення цитозольного перевантаження кальцієм, пригнічення активації зимогенів, стабілізацію мітохондріального метаболізму і зниження вираженості запальної відповіді. Частина з них уже застосовується в інших галузях медицини або проходить клінічні випробування як потенційні засоби терапії ГП. Висновки. Фармакологічна корекція кальцієвого гомеостазу є перспективним патогенетичним підходом до лікування гострого панкреатиту. Існуючі експериментальні й клінічні дані свідчать про доцільність подальших багатоцентрових досліджень для підтвердження ефективності й безпеки таких підходів у рутинній клінічній практиці.
Background. Acute pancreatitis (AP) is a common and potentially life-threatening disease with high rates of complications and mortality. One of its key pathogenic mechanisms is disruption of calcium homeostasis in pancreatic acinar cells. Excessive accumulation of cytosolic calcium triggers premature enzyme activation, mitochondrial dysfunction, oxidative stress, and systemic inflammation. Therefore, development of therapeutic strategies aimed at restoring intracellular calcium balance have emerged as a promising pathogenetic direction. Objective: to analyze current experimental and clinical studies regarding the role of calcium dysregulation in the pathogenesis of AP and to evaluate the efficacy of various approaches to its pharmacological correction. Materials and methods. A literature review was conducted using PubMed, Scopus, and Google Scholar databases up to March 2025. The analysis included experimental (in vitro, in vivo) and clinical studies addressing the mechanisms of calcium imbalance in AP and the effects of different pharmacological agents on its correction. Results. Key molecular targets for calcium-targeted therapy were identified, including IP3 and ryanodine receptors, SOC/CRAC channels, TMEM16A, the PI3K/Akt pathway, and the calcineurin/NFAT signaling cascade. Therapeutic compounds such as caffeine, dantrolene, docosahexaenoic acid, Orai1 channel inhibitors (CM4620/Auxora, GSK-7975A), TMEM16A inhibitors, insulin, calcium chelators (BAPTA-AM), calcineurin inhibitors (cyclosporin A, tacrolimus), and microRNAs (e.g., miR-26a) demonstrated the ability to reduce cytosolic calcium overload, suppress zymogen activation, stabilize mitochondrial function, and attenuate inflammation. Some of these agents are already used in other medical fields or are undergoing clinical trials as candidate treatments for AP. Conclusions. Pharmacological modulation of calcium homeostasis is a promising pathogenetic approach to the treatment of acute pancreatitis. Existing experimental and clinical data support the need for further multicenter studies to confirm the efficacy and safety of these methods in routine clinical practice.
гострий панкреатит; кальцій; кальцієвий гомеостаз; Orai1; інсулін; дантролен; кальциневрин; хелатори кальцію
acute pancreatitis; calcium; calcium homeostasis; Orai1; insulin; dantrolene; calcineurin; calcium chelators
Для ознакомления с полным содержанием статьи необходимо оформить подписку на журнал.
- Mayerle J, Sendler M, Hegyi E, Beyer G, Lerch MM, Sahin-Tth M. Genetics, Cell Biology, and Pathophysiology of Pancreatitis. Gastroenterology. 2019 May;156(7):1951-1968.e1. doi: 10.1053/j.gastro.2018.11.081. Epub 2019 Jan 18. PMID: 30660731.
- Tenner S, Vege SS, Sheth SG, Sauer B, Yang A, Conwell DL, Yadlapati RH, Gardner TB. American College of Gastroenterology Guidelines: Management of Acute Pancreatitis. Am J Gastroenterol. 2024 Mar 1;119(3):419-437. doi: 10.14309/ajg.0000000000002645. Epub 2023 Nov 7. PMID: 38857482.
- Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020 Sep 5;396(10252):726-734. doi: 10.1016/S0140-6736(20)31310-6. PMID: 32891214.
- Mederos MA, Reber HA, Girgis MD. Acute Pancreatitis: A Review. JAMA. 2021 Jan 26;325(4):382-390. doi: 10.1001/jama.2020.20317. PMID: 33496779.
- Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019 Mar;16(3):175-184. doi: 10.1038/s41575-018-0087-5. PMID: 30482911.
- Iannuzzi JP, King JA, Leong JH, Quan J, Windsor JW, Tanyingoh D et al. Global Incidence of Acute Pancreatitis Is Increasing Over Time: A Systematic Review and Meta-Analysis. Gastroenterology. 2022 Jan;162(1):122-134. doi: 10.1053/j.gastro.2021.09.043. Epub 2021 Sep 25. PMID: 34571026.
- Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019 Aug;16(8):479-496. doi: 10.1038/s41575-019-0158-2. PMID: 31138897.
- Li CL, Jiang M, Pan CQ, Li J, Xu LG. The global, regional, and national burden of acute pancreatitis in 204 countries and territories, 1990–2019. BMC Gastroenterol. 2021 Aug 25;21(1):332. doi: 10.1186/s12876-021-01906-2. PMID: 34433418.
- Qiu M, Zhou X, Zippi M, Goyal H, Basharat Z, Jagielski M, Hong W. Comprehensive review on the pathogenesis of hypertriglyceridaemia-associated acute pancreatitis. Ann Med. 2023;55(2):2265939. doi: 10.1080/07853890.2023.2265939. Epub 2023 Oct 9. PMID: 37813108.
- Jia W, Xu L, Xu W, Yang M, Zhang Y. Application of nanotechnology in the diagnosis and treatment of acute pancreatitis. Nanoscale Adv. 2022 Mar 19;4(8):1949-1961. doi: 10.1039/d2na00020b. eCollection 2022 Apr 12. PMID: 36133408.
- Beij A, Verdonk RC, van Santvoort HC, de-Madaria E, Voermans RP. Acute Pancreatitis: An Update of Evidence-Based Management and Recent Trends in Treatment Strategies. United European Gastroenterol J. 2025 Feb;13(1):97-106. doi: 10.1002/ueg2.12743. Epub 2025 Jan 13. PMID: 39804691.tmen Ko D, Bengi G, Gl , zen Alahdab Y, Altnta E,
- Barutu S et al. Turkish Society of Gastroenterology: Pancreas Wor–king Group, Acute Pancreatitis Committee Consensus Report. Turk J Gastroenterol. 2024 Nov 11;35(Suppl 1):S1-S44. doi: 10.5152/tjg.2024.24392. PMID: 39599919.
- Yang J, Tang X, Wu Q, Ren P, Yan Y, Liu W, Pan C. Heparin Protects Severe Acute Pancreatitis by Inhibiting HMGB-1 Active Secretion from Macrophages. Polymers (Basel). 2022 Jun 17;14(12):2470. doi: 10.3390/polym14122470. PMID: 35746047.
- Patil B, Meena LN, Sharma DC, Agarwal G, Dadhich Y, Gupta G. Impact of low-molecular-weight heparin in the treatment of moderately severe and severe acute pancreatitis; a randomized, single blind, phase 3 control trial. Int J Surg. 2022 May;101:106621. doi: 10.1016/j.ijsu.2022.106621. Epub 2022 Apr 27. PMID: 35489648.
- He W, Chen P, Lei Y, Xia L, Liu P, Zhu Y et al. Randomized controlled trial: neostigmine for intra-abdominal hypertension in acute pancreatitis. Crit Care. 2022 Mar 3;26(1):52. doi: 10.1186/s13054-022-03922-4. PMID: 35241135.
- Du W, Wang X, Zhou Y, Wu W, Huang H, Jin Z. From micro to macro, nanotechnology demystifies acute pancreatitis: a new gene-ration of treatment options emerges. J Nanobiotechnology. 2025 Jan 29;23(1):57. doi: 10.1186/s12951-025-03106-6. PMID: 39881355.
- Feng S, Wei Q, Hu Q, Huang X, Zhou X, Luo G, Deng M, L M. Research Progress on the Relationship Between Acute Pancreatitis and Calcium Overload in Acinar Cells. Dig Dis Sci. 2019 Jan;64(1):25-38. doi: 10.1007/s10620-018-5297-8. Epub 2018 Oct 3. PMID: 30284136.
- Wen L, Javed TA, Yimlamai D, Mukherjee A, Xiao X, Husain SZ. Transient High Pressure in Pancreatic Ducts Promotes Inflammation and Alters Tight Junctions via Calcineurin Signaling in Mice. Gastroenterology. 2018 Oct;155(4):1250-1263.e5. doi: 10.1053/j.gastro.2018.06.036. Epub 2018 Jun 19. PMID: 29928898.
- Chvanov M, Voronina S, Jefferson M, Mayer U, Sutton R, Criddle DN, Wileman T, Tepikin AV. Deletion of the WD40 domain of ATG16L1 exacerbates acute pancreatitis, abolishes LAP-like non-canonical autophagy and slows trypsin degradation. Autophagy. 2025 Jan;21(1):210-222. doi: 10.1080/15548627.2024.2392478. Epub 2024 Aug 31. PMID: 39216469.
- Huang W, Cane MC, Mukherjee R, Szatmary P, Zhang X, Elliott V et al. Caffeine protects against experimental acute pancreatitis by inhibition of inositol 1,4,5-trisphosphate receptor-mediated Ca2+ release. Gut. 2017 Feb;66(2):301-313. doi: 10.1136/gutjnl-2015-309363. Epub 2015 Dec 7. PMID: 26642860.
- Chen X, Zhong R, Hu B. Mitochondrial dysfunction in the pathogenesis of acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2025 Feb;24(1):76-83. doi: 10.1016/j.hbpd.2023.12.008. Epub 2023 Dec 30. PMID: 38212158.
- Du W, Liu G, Shi N, Tang D, Ferdek PE, Jakubowska MA et al. A microRNA checkpoint for Ca2+ signaling and overload in acute pancreatitis. Mol Ther. 2022 Apr 6;30(4):1754-1774. doi: 10.1016/j.ymthe.2022.01.033. Epub 2022 Jan 22. PMID: 35077860.
- Petersen OH, Gerasimenko JV, Gerasimenko OV, Gryshchenko O, Peng S. The roles of calcium and ATP in the physiology and patho–logy of the exocrine pancreas. Physiol Rev. 2021 Oct 1;101(4):1691-1744. doi: 10.1152/physrev.00003.2021. Epub 2021 May 5. PMID: 33949875.
- Hu Z, Wang D, Gong J, Li Y, Ma Z, Luo T, Jia X, Shi Y, Song Z. MSCs Deliver Hypoxia-Treated Mitochondria Reprogramming Acinar Metabolism to Alleviate Severe Acute Pancreatitis Injury. Adv Sci (Weinh). 2023 Sep;10(25):e2207691. doi: 10.1002/advs.202207691. Epub 2023 Jul 6. PMID: 37409821.
- Armstrong JA, Cash NJ, Ouyang Y, Morton JC, Chvanov M, Latawiec D et al. Oxidative stress alters mitochondrial bioenergetics and modifies pancreatic cell death independently of cyclophilin D, resulting in an apoptosis-to-necrosis shift. J Biol Chem. 2018 May 25;293(21):8032-8047. doi: 10.1074/jbc.RA118.003200. Epub 2018 Apr 6. PMID: 29626097.
- Sastre J, Prez S, Sabater L, Rius-Prez S. Redox signa–ling in the pancreas in health and disease. Physiol Rev. 2025 Apr 1;105(2):593-650. doi: 10.1152/physrev.00044.2023. Epub 2024 Sep 26. PMID: 39324871.
- Wang Y, Wang X, Zhang X, Zhang B, Meng X, Qian D, Xu Y, Yu L, Yan X, He Z. Inflammation and Acinar Cell Dual-Targeting Nanomedicines for Synergistic Treatment of Acute Pancreatitis via Ca2+ Homeostasis Regulation and Pancreas Autodigestion Inhibition. ACS Nano. 2024 May 7;18(18):11778-11803. doi: 10.1021/acsnano.4c00218. Epub 2024 Apr 23. PMID: 38652869.
- Lopez-Blazquez C, Lacalle-Gonzalez C, Sanz-Criado L, Ochieng’ Otieno M, Garcia-Foncillas J, Martinez-Useros J. Iron-Dependent Cell Death: A New Treatment Approach against Pancreatic Ductal Adenocarcinoma. Int J Mol Sci. 2023 Oct 7;24(19):14979. doi: 10.3390/ijms241914979. PMID: 37834426.
- Biczo G, Vegh ET, Shalbueva N, Mareninova OA, Elperin J, Lotshaw E et al. Mitochondrial Dysfunction, Through Impaired Autophagy, Leads to Endoplasmic Reticulum Stress, Deregulated Lipid Metabolism, and Pancreatitis in Animal Models. Gastroenterology. 2018 Feb;154(3):689-703. doi: 10.1053/j.gastro.2017.10.012. Epub 2017 Oct 23. PMID: 29074451.
- Tong J, Wang Q, Gao Z, Liu Y, Lu C. VMP1: a multifaceted regulator of cellular homeostasis with implications in disease pathology. Front Cell Dev Biol. 2024 Jul 19;12:1436420. doi: 10.3389/fcell.2024.1436420. eCollection 2024. PMID: 39100095.
- Giacomello M, Pyakurel A, Glytsou C, Scorrano L. The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol. 2020 Apr;21(4):204-224. doi: 10.1038/s41580-020-0210-7. Epub 2020 Feb 18. PMID: 32071438.
- Zhang T, Chen S, Li L, Jin Y, Liu S, Liu Z et al. PFKFB3 controls acinar IP3R-mediated Ca2+ overload to regulate acute pancreatitis severity. JCI Insight. 2024 May 23;9(13):e169481. doi: 10.1172/jci.insight.169481. PMID: 38781030.
- Carreras-Sureda A, Pihn P, Hetz C. Calcium signaling at the endoplasmic reticulum: fine-tuning stress responses. Cell Calcium. 2018 Mar;70:24-31. doi: 10.1016/j.ceca.2017.08.004. Epub 2017 Aug 20. PMID: 29054537.
- Song X, Kirtipal N, Lee S, Mal P, Bharadwaj S. Current therapeutic targets and multifaceted physiological impacts of caffeine. Phytother Res. 2023 Dec;37(12):5558-5598. doi: 10.1002/ptr.8000. Epub 2023 Sep 7. PMID: 37679309.
- Zhang X, Jin T, Shi N, Yao L, Yang X, Han C et al. Mechanisms of Pancreatic Injury Induced by Basic Amino Acids Differ Between L-Arginine, L-Ornithine, and L-Histidine. Front Physiol. 2019 Jan 15;9:1922. doi: 10.3389/fphys.2018.01922. eCollection 2018. PMID: 30697165.
- Alazouny ZM, Shaheen MA. The Possible Effects of Caffeine and Nicotine on the Pathogenesis of Experimentally Induced Alcoholic Pancreatitis: A Histological and Immunohistochemical Study. Journal of Medical Histology. 2020;4(1):79-96. doi: 10.21608/jmh.2020.23716.1073.
- Temple JL, Bernard C, Lipshultz SE, Czachor JD, Westphal JA, Mestre MA. The Safety of Ingested Caffeine: A Comprehensive Review. Front Psychiatry. 2017 May 26;8:80. doi: 10.3389/fpsyt.2017.00080. eCollection 2017. PMID: 28603504.
- Nehlig A. Effects of Coffee on the Gastro-Intestinal Tract: A Narrative Review and Literature Update. Nutrients. 2022 Jan 17;14(2):399. doi: 10.3390/nu14020399. PMID: 35057580.
- Saimaiti A, Zhou DD, Li J, Xiong RG, Gan RY, Huang SY et al. Dietary sources, health benefits, and risks of caffeine. Crit Rev Food Sci Nutr. 2023;63(29):9648-9666. doi: 10.1080/10408398.2022.2074362. Epub 2022 May 16. PMID: 35574653.
- Wijarnpreecha K, Panjawatanan P, Mousa OY, Cheungpasitporn W, Pungpapong S, Ungprasert P. Heavy Coffee Consumption and Risk of Pancreatitis: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2018 Nov;63(11):3134-3140. doi: 10.1007/s10620-018-5214-1. Epub 2018 Jul 24. PMID: 30043284.
- Kim SH, Park Y, Lim JW, Kim H. Effect of Docosahexaenoic Acid on Ca2+ Signaling Pathways in Cerulein-Treated Pancreatic Acinar Cells, Determined by RNA-Sequencing Analysis. Nutrients. 2019 Jun 26;11(7):1445. doi: 10.3390/nu11071445. PMID: 31248019.
- Xu P, Wang J, Yang ZW, Lou XL, Chen C. Regulatory roles of the PI3K/Akt signaling pathway in rats with severe acute pancreatitis. PLoS One. 2013 Nov 28;8(11):e81767. doi: 10.1371/journal.pone.0081767. eCollection 2013. PMID: 24312352.
- Murugan AK. mTOR: Role in cancer, metastasis and drug resistance. Semin Cancer Biol. 2019 Dec;59:92-111. doi: 10.1016/j.semcancer.2019.07.003. Epub 2019 Aug 10. PMID: 31408724.
- Liu J, Hu X, Feng L, Lin Y, Liang S, Zhu Z, Shi S, Dong C. Carbonic anhydrase IX-targeted H-APBC nanosystem combined with phototherapy facilitates the efficacy of PI3K/mTOR inhibitor and resists HIF-1-dependent tumor hypoxia adaptation. J Nanobiotechnology. 2022 Apr 12;20(1):187. doi: 10.1186/s12951-022-01394-w. PMID: 35413842.
- Han Y, Shewan AM, Thorn P. HCO3– Transport through Anoctamin/Transmembrane Protein ANO1/TMEM16A in Pancreatic Acinar Cells Regulates Luminal pH. J Biol Chem. 2016 Sep 23;291(39):20345-52. doi: 10.1074/jbc.M116.750224. Epub 2016 Aug 10. PMID: 27510033.
- Yokoyama T, Takemoto M, Hirakawa M, Saino T. Different immunohistochemical localization for TMEM16A and CFTR in acinar and ductal cells of rat major salivary glands and exocrine pancreas. Acta Histochem. 2019 Jan;121(1):50-55. doi: 10.1016/j.acthis.2018.10.013. Epub 2018 Oct 30. PMID: 30389171.
- Wang Q, Bai L, Luo S, Wang T, Yang F, Xia J et al. TMEM16A Ca2+-activated Cl– channel inhibition ameliorates acute pancreatitis via the IP3R/Ca2+/NFB/IL-6 signaling pathway. J Adv Res. 2020 Jan 21;23:25-35. doi: 10.1016/j.jare.2020.01.006. eCollection 2020 May. PMID: 32071789.
- Parness J, Ananthanaravanan M, Bhandari V, Perides G. Ryanodine receptors contribute to bile acid-induced pathological calcium signaling and pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2012 Jun 15;302(12):G1423-33. doi: 10.1152/ajpgi.00546.2011. Epub 2012 Apr 19. PMID: 22517774.
- Orabi AI, Shah AU, Ahmad MU, Choo-Wing R, Parness J, Jain D, Bhandari V, Husain SZ. Dantrolene mitigates caerulein-induced pancreatitis in vivo in mice. Am J Physiol Gastrointest Liver Physiol. 2010 Jul;299(1):G196-204. doi: 10.1152/ajpgi.00498.2009. Epub 2010 May 6. PMID: 20448143.
- Taha AM, Khalid DM, Ezz ME, Gouda MA, El-Shafei AA. The Possible Protective Role of Verapamil and Dantrolene in Experimentally Induced Early Acute Pancreatitis in Male Albino Rat. Egyptian Journal of Histology. 2023;46(3):1360-1375. doi: 10.21608/ejh.2022.140968.1692.
- Vervliet T, Gerasimenko JV, Ferdek PE, Jakubowska MA, Petersen OH, Gerasimenko OV, Bultynck G. BH4 domain peptides derived from Bcl-2/Bcl-XL as novel tools against acute pancreatitis. Cell Death Discov. 2018 May 10;4:58. doi: 10.1038/s41420-018-0054-5. eCollection 2018. PMID: 29760956.
- Jiang B, Liang S, Liang G, Wei H. Could dantrolene be explored as a repurposed drug to treat COVID-19 patients by restoring intracellular calcium homeostasis? Eur Rev Med Pharmacol Sci. 2020 Oct;24(19):10228-10238. doi: 10.26355/eurrev_202010_23247. PMID: 33090434.
- Abreu D, Stone SI, Pearson TS, Bucelli RC, Simpson AN, Hurst S et al. A phase Ib/IIa clinical trial of dantrolene sodium in patients with Wolfram syndrome. JCI Insight. 2021 Aug 9;6(15):e145188. doi: 10.1172/jci.insight.145188. PMID: 34185708.
- Bootman MD, Collins TJ, Peppiatt CM, Prothero LS, Mac-Kenzie L, De Smet P et al. Calcium signalling — an overview. Semin Cell Dev Biol. 2001 Feb;12(1):3-10. doi: 10.1006/scdb.2000.0211. PMID: 11162741.
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000 Oct;1(1):11-21. doi: 10.1038/35036035. PMID: 11413485.
- Balla T. Ca2+ and lipid signals hold hands at endoplasmic reticulum-plasma membrane contact sites. J Physiol. 2018 Jul;596(14):2709-2716. doi: 10.1113/JP274957. Epub 2018 Jan 4. PMID: 29210464.
- Englisch CN, Kirstein E, Diebolt CM, Wagner M, Tschernig T. Distribution of TRPC3 and TRPC6 in the human exocrine and endocrine pancreas. Pathol Res Pract. 2024 Aug;260:155403. doi: 10.1016/j.prp.2024.155403. Epub 2024 Jun 10. PMID: 38870712.
- Pallagi P, Madcsy T, Varga , Malth J. Intracellular Ca2+ Signalling in the Pathogenesis of Acute Pancreatitis: Recent Advances and Translational Perspectives. Int J Mol Sci. 2020 Jun 3;21(11):4005. doi: 10.3390/ijms21114005. PMID: 32503336.
- Manhas N. Computational Model of Complex Calcium Dynamics: Store Operated Ca2+ Channels and Mitochondrial Associated Membranes in Pancreatic Acinar Cells. Cell Biochem Biophys. 2025 Mar;83(1):519-535. doi: 10.1007/s12013-024-01484-6. Epub 2024 Sep 13. PMID: 39266873.
- Sabourin J, Le Gal L, Saurwein L, Haefliger JA, Raddatz E, Allagnat F. Store-operated Ca2+ Entry Mediated by Orai1 and TRPC1 Participates to Insulin Secretion in Rat -Cells. J Biol Chem. 2015 Dec 18;290(51):30530-9. doi: 10.1074/jbc.M115.682583. Epub 2015 Oct 22. PMID: 26494622.
- Kim MS, Hong JH, Li Q, Shin DM, Abramowitz J, Birnbaumer L, Muallem S. Deletion of TRPC3 in mice reduces store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology. 2009 Oct;137(4):1509-17. doi: 10.1053/j.gastro.2009.07.042. Epub 2009 Jul 19. PMID: 19622358.
- Wang G, Zhang J, Xu C, Han X, Gao Y, Chen H. Inhibition of SOCs Attenuates Acute Lung Injury Induced by Severe Acute Pancreatitis in Rats and PMVECs Injury Induced by Lipopolysaccharide. Inflammation. 2016 Jun;39(3):1049-58. doi: 10.1007/s10753-016-0335-1. PMID: 27025854.
- Zhang X, Xin P, Yoast RE, Emrich SM, Johnson MT, Pathak T et al. Distinct pharmacological profiles of ORAI1, ORAI2, and ORAI3 channels. Cell Calcium. 2020 Nov;91:102281. doi: 10.1016/j.ceca.2020.102281. Epub 2020 Aug 29. PMID: 32896813.
- De Moudt S, De Munck D, Coornaert I, Fransen P. GSK-7975A, an inhibitor of Ca(2+) release-activated calcium channels, depresses isometric contraction of mouse aorta. Eur J Pharmacol. 2021 Sep 5;906:174197. doi: 10.1016/j.ejphar.2021.174197. Epub 2021 May 27. PMID: 34052216.
- Wen L, Voronina S, Javed MA, Awais M, Szatmary P, Latawiec D et al. Inhibitors of ORAI1 Prevent Cytosolic Calcium-Associated Injury of Human Pancreatic Acinar Cells and Acute Pancreatitis in 3 Mouse Models. Gastroenterology. 2015 Aug;149(2):481-92.e7. doi: 10.1053/j.gastro.2015.04.015. Epub 2015 Apr 25. PMID: 25917787.
- Waldron RT, Chen Y, Pham H, Go A, Su HY, Hu C et al. The Orai Ca2+ channel inhibitor CM4620 targets both parenchymal and immune cells to reduce inflammation in experimental acute pancreatitis. J Physiol. 2019 Jun;597(12):3085-3105. doi: 10.1113/JP277856. Epub 2019 May 22. PMID: 31050811.
- Lewis S, Evans DL, Tsugorka TT, Peng S, Stauderman K, Gerasimenko O, Gerasimenko J. Combination of the CRAC Channel Inhibitor CM4620 and Galactose as a Potential Therapy for Acute Pancreatitis. Function (Oxf). 2024 Jul 11;5(4):zqae017. doi: 10.1093/function/zqae017. PMID: 38984998.
- Bruen C, Miller J, Wilburn J, Mackey C, Bollen TL, Stauderman K, Hebbar S. Auxora for the Treatment of Patients With Acute Pancreatitis and Accompanying Systemic Inflammatory Response Syndrome: Clinical Development of a Calcium Release-Activated Calcium Channel Inhibitor. Pancreas. 2021 Apr 1;50(4):537-543. doi: 10.1097/MPA.0000000000001793. PMID: 33939666.
- A Study of Auxora in Patients With Acute Pancreatitis and Accompanying SIRS (CARPO). Available at: https://clinicaltrials.gov/study/NCT04681066. Accessed 22 July 2024.
- Miller J, Bruen C, Schnaus M, Zhang J, Ali S, Lind A et al. Auxora versus standard of care for the treatment of severe or critical COVID-19 pneumonia: results from a randomized controlled trial. Crit Care. 2020 Aug 14;24(1):502. doi: 10.1186/s13054-020-03220-x. PMID: 32795330.
- Bruen C, Al-Saadi M, Michelson EA, Tanios M, Mendoza-Ayala R, Miller J et al. Auxora vs. placebo for the treatment of patients with severe COVID-19 pneumonia: a randomized-controlled clinical trial. Crit Care. 2022 Apr 8;26(1):101. doi: 10.1186/s13054-022-03964-8. PMID: 35395943.
- Pallagi P, Grg M, Papp N, Madcsy T, Varga , Crul T et al. Bile acid- and ethanol-mediated activation of Orai1 damages pancreatic ductal secretion in acute pancreatitis. J Physiol. 2022 Apr;600(7):1631-1650. doi: 10.1113/JP282203. Epub 2022 Feb 17. PMID: 35081662.
- Gerasimenko OV, Gerasimenko JV. CRAC channel inhibitors in pancreatic pathologies. J Physiol. 2022 Apr;600(7):1597-1598. doi: 10.1113/JP282826. Epub 2022 Mar 16. PMID: 35218580.
- Romac JM, Shahid RA, Swain SM, Vigna SR, Liddle RA. Piezo1 is a mechanically activated ion channel and mediates pressure induced pancreatitis. Nat Commun. 2018 Apr 30;9(1):1715. doi: 10.1038/s41467-018-04194-9. PMID: 29712913.
- Goyal N, Skrdla P, Schroyer R, Kumar S, Fernando D, Oughton A et al. Clinical Pharmacokinetics, Safety, and Tolerability of a Novel, First-in-Class TRPV4 Ion Channel Inhibitor, GSK2798745, in Healthy and Heart Failure Subjects. Am J Cardiovasc Drugs. 2019 Jun;19(3):335-342. doi: 10.1007/s40256-018-00320-6. PMID: 30637626.
- Thien ND, Hai-Nam N, Anh DT, Baecker D. Piezo1 and its inhibitors: Overview and perspectives. Eur J Med Chem. 2024 Jul 5;273:116502. doi: 10.1016/j.ejmech.2024.116502. Epub 2024 May 14. PMID: 38761789.
- Zhang Q, Zhao C, Zhang L, Sun K, Yu L, Wang X et al. Escin Sodium Improves the Prognosis of Acute Pancreatitis via Promoting Cell Apoptosis by Suppression of the ERK/STAT3 Signaling Pathway. Oxid Med Cell Longev. 2021 Aug 12;2021:9921839. doi: 10.1155/2021/9921839. eCollection 2021. PMID: 34422214.
- Eisner D, Neher E, Taschenberger H, Smith G. Physiology of intracellular calcium buffering. Physiol Rev. 2023 Oct 1;103(4):2767-2845. doi: 10.1152/physrev.00042.2022. Epub 2023 Jun 16. PMID: 37326298.
- Zhu W, Takeuchi S, Imai S, Terada T, Ueda T, Nasu Y, Terai T, Campbell RE. Chemigenetic indicators based on synthetic chelators and green fluorescent protein. Nat Chem Biol. 2023 Jan;19(1):38-44. doi: 10.1038/s41589-022-01134-z. Epub 2022 Sep 22. PMID: 36138142.
- Fu Z, Wang D, Zheng C, Xie M, Chen Y, Zhou Y et al. Elimination of intracellular Ca2+ overload by BAPTA AM liposome nanoparticles: A promising treatment for acute pancreatitis. Int J Mol Med. 2024 Apr;53(4):34. doi: 10.3892/ijmm.2024.5358. Epub 2024 Feb 23. PMID: 38390952.
- Luo T, Tang Y, Xie W, Ma Z, Gong J, Zhang Y et al. Cerium-based nanoplatform for severe acute pancreatitis: Achieving enhanced anti-inflammatory effects through calcium homeostasis restoration and oxidative stress mitigation. Mater Today Bio. 2025 Jan 13;31:101489. doi: 10.1016/j.mtbio.2025.101489. eCollection 2025 Apr. PMID: 39906206.
- Gerasimenko JV, Gerasimenko OV. The role of Ca2+ signalling in the pathology of exocrine pancreas. Cell Calcium. 2023 Jun;112:102740. doi: 10.1016/j.ceca.2023.102740. Epub 2023 Apr 8. PMID: 37058923.
- Peng S, Gerasimenko JV, Tsugorka TM, Gryshchenko O, Samarasinghe S, Petersen OH, Gerasimenko OV. Galactose protects against cell damage in mouse models of acute pancreatitis. J Clin Invest. 2018 Aug 31;128(9):3769-3778. doi: 10.1172/JCI94714. Epub 2018 Jul 30. PMID: 29893744.
- Wong SY, Gadomski T, van Scherpenzeel M, Honzik T, Hansikova H, Holmefjord KSB et al. Oral D-galactose supplementation in PGM1-CDG. Genet Med. 2017 Nov;19(11):1226-1235. doi: 10.1038/gim.2017.41. Epub 2017 Jun 15. PMID: 28617415.
- Witters P, Andersson H, Jaeken J, Tseng L, van Karnebeek CDM, Lefeber DJ, Cassiman D, Morava E. D-galactose supplementation in individuals with PMM2-CDG: results of a multicenter, open label, prospective pilot clinical trial. Orphanet J Rare Dis. 2021 Mar 20;16(1):138. doi: 10.1186/s13023-020-01609-z. PMID: 33743737.
- Mik A, Erss B, Sarls P, Hegyi P Jr, Mrta K, Pcsi D et al. Observational longitudinal multicentre investigation of acute pancreatitis (GOULASH PLUS): follow-up of the GOULASH study, protocol. BMJ Open. 2019 Sep 3;9(8):e025500. doi: 10.1136/bmjopen-2018-025500. PMID: 31481363.
- Samad A, James A, Wong J, Mankad P, Whitehouse J, Patel W, Alves-Simoes M, Siriwardena AK, Bruce JI. Insulin protects pancreatic acinar cells from palmitoleic acid-induced cellular injury. J Biol Chem. 2014 Aug 22;289(34):23582-95. doi: 10.1074/jbc.M114.589440. Epub 2014 Jul 3. PMID: 24993827.
- Bruce JIE, Snchez-Alvarez R, Sans MD, Sugden SA, Qi N, James AD, Williams JA. Insulin protects acinar cells during pancreatitis by preserving glycolytic ATP supply to calcium pumps. Nat Commun. 2021 Jul 19;12(1):4386. doi: 10.1038/s41467-021-24506-w. PMID: 34282152.
- Wen L, Husain SZ. Endogenous insulin directly protects pancreatic acinar cells in pancreatitis. Cell Calcium. 2021 Dec;100:102485. doi: 10.1016/j.ceca.2021.102485. Epub 2021 Oct 11. PMID: 34655986.
- Vasques ER, Cunha JEM, Kubrusly MS, Coelho AM, Sanpietri SN, Nader HB et al. The M-RNA, expression of SERCA2 and NCX1 in the process of pharmacological cell protection in experimental acute pancreatitis induced by taurocholate. Arq Bras Cir Dig. 2018 Jun 21;31(1):e1352. doi: 10.1590/0102-672020180001e1352. PMID: 29947686.
- Zhao T, Fang R, Ding J, Liu Y, Cheng M, Zhou F et al. Melatonin ameliorates multiorgan injuries induced by severe acute pancreatitis in mice by regulating the Nrf2 signaling pathway. Eur J Pharmacol. 2024 Jul 15;975:176646. doi: 10.1016/j.ejphar.2024.176646. Epub 2024 May 17. PMID: 38762157.
- Schick V, Scheiber JA, Mooren FC, Turi S, Ceyhan GO, Schnekenburger J et al. Effect of magnesium supplementation and depletion on the onset and course of acute experimental pancreatitis. Gut. 2014 Sep;63(9):1469-80. doi: 10.1136/gutjnl-2012-304274. Epub 2013 Nov 25. PMID: 24277728.
- Aletaha N, Hamid H, Alipour A, Ketabi Moghadam P. Magnesium Sulfate for Prevention of Post-ERCP-Pancreatitis: A Rando–mized Controlled Trial. Arch Iran Med. 2022 Mar 1;25(3):148-154. doi: 10.34172/aim.2022.25. PMID: 35429955.
- Wen L, Javed TA, Dobbs AK, Brown R, Niu M, Li L et al. The Protective Effects of Calcineurin on Pancreatitis in Mice Depend on the Cellular Source. Gastroenterology. 2020 Sep;159(3):1036-1050.e8. doi: 10.1053/j.gastro.2020.05.051. Epub 2020 May 20. PMID: 32445858.
- Muili KA, Wang D, Orabi AI, Sarwar S, Luo Y, Javed TA et al. Bile acids induce pancreatic acinar cell injury and pancreatitis by activating calcineurin. J Biol Chem. 2013 Jan 4;288(1):570-80. doi: 10.1074/jbc.M112.428896. Epub 2012 Nov 12. PMID: 23148215.
- Choi JW, Shin J, Zhou Z, Song HJ, Bae GS, Kim MS, Park SJ. Myricetin ameliorates the severity of pancreatitis in mice by regulating cathepsin B activity and inflammatory cytokine production. Int Immunopharmacol. 2024 Jul 30;136:112284. doi: 10.1016/j.intimp.2024.112284. Epub 2024 May 31. PMID: 38823179.
- Karolin A, Genitsch V, Sidler D. Calcineurin Inhibitor Toxi-city in Solid Organ Transplantation. Pharmacology. 2021;106(7-8):347-355. doi: 10.1159/000515933. Epub 2021 Jun 15. PMID: 34130291.
- Farouk SS, Rein JL. The Many Faces of Calcineurin Inhibitor Toxicity-What the FK? Adv Chronic Kidney Dis. 2020 Jan;27(1):56-66. doi: 10.1053/j.ackd.2019.08.006. PMID: 32147003.
- Lerch MM, Aghdassi AA, Sendler M. Cell Signaling of Pancreatic Duct Pressure and Its Role in the Onset of Pancreatitis. Gastroenterology. 2020 Sep;159(3):827-831. doi: 10.1053/j.gastro.2020.07.027. Epub 2020 Jul 18. PMID: 32693183.
- Ni J, Khalid A, Lin YC, Barakat MT, Wang J, Tsai CY, Azar PRS et al. Preclinical safety evaluation of calcineurin inhibitors delivered through an intraductal route to prevent post-ERCP pancreatitis demonstrates endocrine and systemic safety. Pancreatology. 2023 Jun;23(4):333-340. doi: 10.1016/j.pan.2023.03.009. Epub 2023 Mar 30. PMID: 37031049.
- Orabi AI, Wen L, Javed TA, Le T, Guo P, Sanker S et al. Targeted inhibition of pancreatic acinar cell calcineurin is a novel strategy to prevent post-ERCP pancreatitis. Cell Mol Gastroenterol Hepatol. 2017 Jan;3(1):119-128. doi: 10.1016/j.jcmgh.2016.08.006. PMID: 28090570.
- Barakat MT, Khalid A, Yu M, Ding Y, Murayi JA, Jayaraman T et al. A review of the rationale for the testing of the calcineurin inhibitor tacrolimus for post-ERCP pancreatitis prevention. Pancreato–logy. 2022 Sep;22(6):678-682. doi: 10.1016/j.pan.2022.07.003. Epub 2022 Jul 13. PMID: 35872075.
- Rao BH, Vincent PK, Nair P, Koshy AK, Venu RP. Preventive effect of tacrolimus on patients with post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Endosc. 2022 Sep;55(5):665-673. doi: 10.5946/ce.2021.265. Epub 2022 Aug 2. PMID: 35915049.
- Parlakpinar H, Gunata M. Transplantation and immunosuppression: a review of novel transplant-related immunosuppressant drugs. Immunopharmacol Immunotoxicol. 2021 Dec;43(6):651-665. doi: 10.1080/08923973.2021.1966033. Epub 2021 Aug 20. PMID: 34415233.
- Lin YC, Ni J, Swaminathan G, Khalid A, Barakat MT, Frymoyer AR, Tsai CY et al. Rectal administration of tacrolimus protects against post-ERCP pancreatitis in mice. Pancreatology. 2023 Nov;23(7):777-783. doi: 10.1016/j.pan.2023.09.080. Epub 2023 Sep 24. PMID: 37778935.
- Stifft F, Vanmolkot F, Scheffers I, van Bortel L, Neef C, Christiaans M. Rectal and sublingual administration of tacrolimus: a single-dose pharmacokinetic study in healthy volunteers. Br J Clin Pharmacol. 2014 Nov;78(5):996-1004. doi: 10.1111/bcp.12420. PMID: 24809233.
- Akshintala VS, Husain SZ, Brenner TA, Singh A, Singh VK, Khashab MA et al. Rectal INdomethacin, oral TacROlimus, or their combination for the prevention of post-ERCP pancreatitis (INTRO Trial): Protocol for a randomized, controlled, double-blinded trial. Pancreatology. 2022 Nov;22(7):887-893. doi: 10.1016/j.pan.2022.07.008. Epub 2022 Jul 19. PMID: 35872074.
- Thiruvengadam NR, Kochman ML. Emerging Therapies to Prevent Post-ERCP Pancreatitis. Curr Gastroenterol Rep. 2020 Nov 13;22(12):59. doi: 10.1007/s11894-020-00796-w. PMID: 33188441.