Международный эндокринологический журнал Том 21, №4, 2025
Вернуться к номеру
Діабетичний дистрес, хронічне запалення низької інтенсивності і кардіальна автономна нейропатія у внутрішньо переміщених осіб (огляд літератури і власні спостереження)
Авторы: Сергієнко В.О.
Львівський національний медичний університет імені Данила Галицького, м. Львів, Україна
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
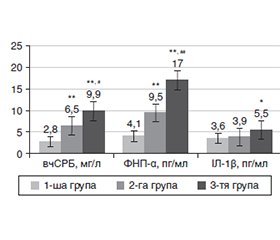
Актуальність. Внутрішньо переміщені особи (ВПО) демонструють підвищену сприйнятливість до виникнення серцево-судинних захворювань і цукрового діабету (ЦД) 2-го типу. Негативний психотравматичний досвід може спричинити розвиток емоційних розладів, які додатково сприяють виникненню діабетичного дистресу (ДДС). Психосоціальні фактори ризику ДДС значною мірою пов’язані із загрозою розвитку кардіальної автономної нейропатії (КАН), а отже, ризиком несприятливих серцево-судинних подій. Система оцінки дистресу при ЦД 2-го типу (The Type 2 Diabetes Distress Assessment System, T2-DDAS) — єдиний спеціалізований інструмент оцінки ДДС. Мета дослідження: за допомогою використання валідованої нами україномовної версії T2-DDAS встановити рівень ДДС серед ВПО. Матеріали та методи. Опрацювання і застосування нами валідованої україномовної версії опитувальника «Композитна оцінка вегетативних симптомів 31» (Composite Autonomic Symptom Score, COMPASS 31) продемонструвало, що COMPASS 31 є прийнятним інструментом для виокремлення пацієнтів із субклінічною КАН. Результати. Проведений нами аналіз результатів короткочасної варіабельності серцевого ритму (ВСР) свідчить про те, що у ВПО, хворих на ЦД 2-го типу, спостерігається більш значне пригнічення активності парасимпатичної (ПСНС) і посилення симпатичної нервової системи (СНС). Це може свідчити про адаптаційні реакції, що виникли на фоні стресових ситуацій. Крім того, зафіксовано зміни ВСР, типові для станів інтенсивного нервово-емоційного напруження, що може свідчити про тривалу активацію СНС. Виявлені зміни вказують на раннє порушення стану ПСНС, що є однією з перших ознак розвитку КАН при ЦД 2-го типу. Психологічний стрес має здатність активувати СНС, що своєю чергою сприяє розвитку хронічного запалення низької інтенсивності (ХЗНІ). У ВПО, хворих на ЦД 2-го типу із субклінічною КАН, виявлено найбільш виражену ІР. Крім того, спостерігалося значне підвищення рівня високочутливого С-реактивного білка, фактора некрозу пухлини α (ФНП-α), а також співвідношення ФНП-α/інтерлейкін-10 у крові. Висновки. Вимірювання короткочасної ВСР у поєднанні із визначенням рівнів прозапальних маркерів надає можливість більш точно оцінити ризик розвитку КАН у пацієнтів із ЦД 2-го типу.
Background. Internally displaced people (IDP) demonstrate an increased susceptibility to cardiovascular diseases and type 2 diabetes mellitus (T2DM). Negative psycho-traumatic experiences can lead to the development of emotional disorders, which further contribute to the onset of diabetic distress (DD). Psychosocial risk factors for DD are largely associated with the risk of developing cardiac autonomic neuropathy (CAN) and, thus, adverse cardiovascular events. The Type 2 Diabetes Distress Assessment System (T2-DDAS) is the only specialized tool for assessing DD. The purpose: using the Ukrainian version of the T2-DDAS validated by us to assess the level of DD among IDP. Materials and methods. Development and application of a validated Ukrainian-language version of the Composite Autonomic Symptom Score 31 demonstrated that this is an acceptable tool for identifying patients with subclinical CAN. Results. Analysis of the results of short-term heart rate variability (HRV) shows that IDP with T2DM have a more significant suppression of parasympathetic nervous system (PSNS) activity and an increase in sympathetic nervous system (SNS) activity. This may indicate adaptive reactions that occurred against the background of stressful situations. In addition, HRV changes typical of states of intense neuro-emotional stress were recorded, which may suggest prolonged activation of the SNS. The changes detected indicate an early disturbance of the PSNS, which is one of the first signs of CAN in T2DM. Psychological stress can activate the SNS, which in turn contributes to the development of low-grade chronic inflammation. We have found that IDP with T2DM and subclinical CAN had the most pronounced insulin resistance. In addition, there was a significant increase in the level of high-sensitivity C-reactive protein, tumor necrosis factor α, as well as the tumor necrosis factor α/interleukin 10 ratio in the blood. Conclusions. Thus, the measurement of short-term HRV in combination with the determination of proinflammatory marker levels provides an opportunity to more accurately assess the risk of developing CAN in patients with T2DM.
внутрішньо переміщені особи; цукровий діабет 2-го типу; діабетичний дистрес; кардіальна автономна нейропатія; короткочасна варіабельність серцевого ритму; хронічне запалення низької інтенсивності; огляд
internally displaced people; type 2 diabetes mellitus; diabetic distress; cardiac autonomic neuropathy; short-term heart rate variability; low-grade chronic inflammation; review
Для ознакомления с полным содержанием статьи необходимо оформить подписку на журнал.
- Pankiv V, Yuzvenko T, Vasiuk V, Nykytiuk L, Yuzvenko V, Mikulets L. Association between diabetes distress and sociodemographic factors among adults in Ukraine. Mìžnarodnij endokrinologìčnij žurnal. 2024;20(5):394-399. doi: 10.22141/2224-0721.20.5.2024.1426.
- Vesco P, Balikic G, Tilman Brückc T, et al. The impacts of armed conflict on human development: A review of the literature. World Dev. 2025;187:106806. doi: 10.1016/j.worlddev.2024.106806.
- Yang Y, Niu L, Amin S, Yasin I. Unemployment and mental health: A global study of unemployment’s influence on diverse mental disorders. Front Public Health. 2024 Dec 13;12:1440403. doi: 10.3389/fpubh.2024.1440403.
- Topluoglu S, Taylan-Ozkan A, Alp E. Impact of wars and natural disasters on emerging and re-emerging infectious disea–ses. Front Public Health. 2023 Sep 1;11:1215929. doi: 10.3389/fpubh.2023.1215929.
- Verme P, Schuettler K. The impact of forced displacement on host communities: A review of the empirical literature in economics. J. Dev. Econ. 2021;150:102606. doi: 10.1016/j.jdeveco.2020.102606.
- Ali AMA, Adam OAM, Salem SEM, et al. Prevalence of physi–cal and mental health problems among internally displaced persons in White Nile state, Sudan 2023: A cross sectional study. BMC Public Health. 2024 Dec 18;24(1):3448. doi: 10.1186/s12889-024-20972-1.
- Arage MW, Kumsa H, Asfaw MS, et al. Exploring the health consequences of armed conflict: The perspective of Northeast Ethio–pia, 2022: A qualitative study. BMC Public Health. 2023 Oct 24;23(1):2078. doi: 10.1186/s12889-023-16983-z.
- Sh Abukar IM, Asir Rage AA, Warsame MO. Prevalence of post-traumatic stress disorder and associated factors among internally displaced persons (IDPS) in Mogadishu Cross-Sectional Study. Psychol Res Behav Manag. 2025 Jan 25;18:183-196. doi: 10.2147/PRBM.S488388.
- Greene-Cramer B, Summers A, Lopes-Cardozo B, Husain F, Couture A, Bilukha O. Noncommunicable disease burden among conflict-affected adults in Ukraine: A cross-sectional study of prevalence, risk factors, and effect of conflict on severity of disease and access to care. PLoS One. 2020 Apr 21;15(4):e0231899. doi: 10.1371/journal.pone.0231899.
- Pandey A, Wells CR, Stadnytskyi V, et al. Disease burden among Ukrainians forcibly displaced by the 2022 Russian invasion. Proc Natl Acad Sci U S A. 2023 Feb 21;120(8):e2215424120. doi: 10.1073/pnas.2215424120.
- Serhiyenko VA, Sehin VB, Serhiyenko AA. Effect of forced resettlement on chronic inflammation and insulin resistance in diabetic cardiac autonomic neuropathy. Problemi Endocrinnoi Patologii. 2025;82(1):33-39. doi: 10.21856/j-PEP.2025.1.04.
- Kirkbride JB, Anglin DM, Colman I, et al. The social determinants of mental health and disorder: evidence, prevention and recommendations. World Psychiatry. 2024 Feb;23(1):58-90. doi: 10.1002/wps.21160.
- Shinan-Altman S. Challenges faced by internally displaced diabetes patients in managing their health during a conflict: a qualitative study. Confl Health. 2024 Oct 16;18(1):60. doi: 10.1186/s13031-024-00625-1.
- Coccaro EF, Drossos T, Kline D, Lazarus S, Joseph JJ, de Groot M. Diabetes distress, emotional regulation, HbA1c in people with diabetes and a controlled pilot study of an emotion-focused behavioral therapy intervention in adults with type 2 diabetes. Prim Care Diabetes. 2022 Jun;16(3):381-386. doi: 10.1016/j.pcd.2022.03.002.
- Morales-Brown LA, Perez Algorta G, Salifu Y. Understan–ding experiences of diabetes distress: A systematic review and thematic synthesis. J Diabetes Res. 2024 Nov 14;2024:3946553. doi: 10.1155/2024/3946553.
- Skinner TC, Joensen L, Parkin T. Twenty-five years of dia–betes distress research. Diabet Med. 2020 Mar;37(3):393-400. doi: 10.1111/dme.14157.
- Fayed A, AlRadini F, Alzuhairi RM, et al. Relation between diabetes related distress and glycemic control: The mediating effect of adherence to treatment. Prim Care Diabetes. 2022 Apr;16(2):293-300. doi: 10.1016/j.pcd.2021.12.004.
- Grulovic N, Rojnic Kuzman M, Baretic M. Prevalence and predictors of diabetes-related distress in adults with type 1 diabetes. Sci Rep. 2022 Sep 21;12(1):15758. doi: 10.1038/s41598-022-19961-4.
- Zhang YY, Li W, Sheng Y, Wang Q, Zhao F, Wei Y. Prevalence and correlators of diabetes distress in adults with type 2 diabetes: A cross-sectional study. Patient Prefer Adherence. 2024 Jan 13;18:111-130. doi: 10.2147/PPA.S442838.
- Wojujutari AK, Idemudia ES, Ugwu LE. Psychological resi–lience mediates the relationship between diabetes distress and depression among persons with diabetes in a multi-group analysis. Sci Rep. 2024 Mar 18;14(1):6510. doi: 10.1038/s41598-024-57212-w.
- Liu X. Advances in psychological and social aetiology of patients with diabetes. Diabetes Metab Syndr Obes. 2023 Dec 23;16:4187-4194. doi: 10.2147/DMSO.S439767.
- Mangoulia P, Milionis C, Vlachou E, Ilias I. The interrelationship between diabetes mellitus and emotional well-being: current concepts and future prospects. Healthcare (Basel). 2024 Jul 22;12(14):1457. doi: 10.3390/healthcare12141457.
- Porth AK, Seidler Y, Long PA, et al. Putting person-centred psychosocial diabetes care into practice: two psychosocial care pathways based on outcome preferences of people with diabetes and healthcare professionals. BMJ Ment Health. 2024 Aug 25;27(1):e301061. doi: 10.1136/bmjment-2024-301061.
- Fisher L, Polonsky WH, Perez-Nieves M, Desai U, Stryc–ker L, Hessler D. A new perspective on diabetes distress using the type 2 diabetes distress assessment system (T2-DDAS): prevalence and change over time. J Diabetes Complications. 2022 Aug;36(8):108256. doi: 10.1016/j.jdiacomp.2022.108256.
- Serhiyenko A, Baitsar M, Sehin V, Serhiyenko L, Kuznets V, Serhiyenko V. Post-traumatic stress disorder, insomnia, heart rate variability and metabolic syndrome (narrative review). Proc Shevchenko Sci Soc Med Sci. 2024 Jun;73(1):1-10. doi: 10.25040/ntsh2024.01.07.
- Wu X, Zu Y, Li D, Yoshida Y. Psychosocial and behavioral risk patterns and risk of cardiovascular complications in people with type 2 diabetes. Diabetes Res Clin Pract. 2025 Mar;221:112037. doi: 10.1016/j.diabres.2025.112037.
- Serhiyenko V, Serhiyenko A. Diabetic Cardiac Autonomic Neuropathy. In: Rodriguez-Saldana JR, editor. The Diabetes Textbook: Clinical Principles, Patient Management and Public Health Issues. 2nd ed. Basel: Springer, Cham: Springer Nature Switzerland AG. 2023. 939-966 pp. doi: 10.1007/978-3-031-25519-9_57.
- Hoogendoorn CJ, Krause-Steinrauf H, Uschner D, et al.; GRADE Research Group. Emotional distress predicts reduced type 2 diabetes treatment adherence in the glycemia reduction approaches in diabetes: A comparative effectiveness study (GRADE). Diabetes Care. 2024 Apr 1;47(4):629-637. doi: 10.2337/dc23-1401.
- Polonsky WH, Fisher L, Hessler D, Desai U, King SB, Perez-Nieves M. Toward a more comprehensive understanding of the emotional side of type 2 diabetes: A re-envisioning of the assessment of diabetes distress. J Diabetes Complications. 2022 Jan;36(1):108103. doi: 10.1016/j.jdiacomp.2021.108103.
- Serhiyenko VA, Sehin VB, Serhiyenko AA. Questionnaire “Composite assessment of autonomic symptoms 31” (COMPASS 31) validation and possibilities of application in the diagnostics of autono–mic dysfunction in patients with type 2 diabetes mellitus. Endokrynologia. 2024;29(4):338-346. doi: 10.31793/1680-1466.2024.29-4.338.
- Sharma B, Tripathi P, Kadam N, et al. Psychometric vali–dation of Type 2 Diabetes Distress Assessment System in an Indian type 2 diabetes population. Sci Rep. 2024;14:32059. doi: 10.1038/s41598-024-83681-0.
- Serhiyenko VA, Sehin VB, Tsaryk TV, Holovach SY, Ser–hiyenko AA. Diabetic distress: the main etiopathogenetic factors and psychometric methods of diagnosis. Lviv-Toruń: Liha-Pres. 2025. 112 p. doi: 10.36059/978-966-397-492-7.
- Aguilar M, Alberti K, Amiel SA, et al.; 1999 European Diabetes Policy Group. A desktop guide to Type 2 diabetes mellitus. Diabet Med. 1999 Sep;16(9):716-730. doi: 10.1046/j.1464-5491.1999.00166.x.
- Spallone V. Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: what is defined, what is new, and what is unmet. Diabetes Metab J. 2019 Feb;43(1):3-30. doi: 10.4093/dmj.2018.0259.
- Williams S, Raheim SA, Khan MI, et al. Cardiac autonomic neuropathy in type 1 and 2 diabetes: epidemiology, pathophysiology, and management. Clin Ther. 2022 Oct;44(10):1394-1416. doi: 10.1016/j.clinthera.2022.09.002.
- Serhiyenko VA, Serhiyenko LM, Sehin VB, Serhiyenko AA. Effect of alpha-lipoic acid on arterial stiffness parameters in type 2 diabetes mellitus patients with cardiac autonomic neuropathy. Endocr Regul. 2021;55(4): 224-233. doi: 10.2478/enr-2021-0024.
- Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: A refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc. 2012 Dec;87(12):1196-1201. doi: 10.1016/j.mayocp.2012.10.013.
- Zhang Z, Ma Y, Fu L, et al. Combination of Composite Autonomic Symptom Score 31 and heart rate variability for diagnosis of cardiovascular autonomic neuropathy in people with type 2 diabetes. J Diabetes Res. 2020 Oct 30;2020:5316769. doi: 10.1155/2020/5316769.
- Meling S, Tjora E, Eichele H, et al. The Composite Autonomic Symptom Score 31 questionnaire: A sensitive test to detect risk for autonomic neuropathy. J Diabetes Res. 2023 Aug 9;2023:4441115. doi: 10.1155/2023/4441115.
- Atala YB, De Matos MR, Zantut-Wittmann DE, et al. Cardiovascular autonomic reflex tests and 7 heart rate variability indices for early diagnosis of cardiovascular autonomic neuropathy in type 2 diabetes individuals. Curr Diabetes Rev. 2022;18(4):e270821195908. doi: 10.2174/1573399817666210827130339.
- Giunta S, Xia S, Pelliccioni G, Olivieri F. Autonomic nervous system imbalance during aging contributes to impair endogenous anti-inflammaging strategies. Geroscience. 2024 Feb;46(1):113-127. doi: 10.1007/s11357-023-00947-7.
- Agorastos A, Mansueto AC, Hager T, Pappi E, Gardikioti A, Stiedl O. Heart rate variability as a translational dynamic biomarker of altered autonomic function in health and psychiatric disease. Biomedicines. 2023 May 30;11(6):1591. doi: 10.3390/biomedicines11061591.
- Serhiyenko VA, Serhiyenko AA. Ezetimibe and diabetes mellitus: a new strategy for lowering cholesterol. Mìžnarodnij endokrinologìčnij žurnal (Ukraine). 2022;18(5):63-75. doi: 10.22141/2224-0721.18.5.2022.1190.
- Ajoolabady A, Pratico D, Vinciguerra M, Lip GYH, Franceschi C, Ren J. Inflammaging: mechanisms and role in the cardiac and vasculature. Trends Endocrinol Metab. 2023 Jun;34(6):373-387. doi: 10.1016/j.tem.2023.03.005.
- Serhiyenko VA, Serhiyenko LM, Serhiyenko AA. Features of Circadian Rhythms of Heart Rate Variability, Arterial Stiffness and Outpatient Monitoring of Blood Pressure in Diabetes Mellitus: Data, Mechanisms and Consequences. In: Sinha RP, editor. Circadian Rhythms and Their Importance. New York, NY: Nova Science Publi–shers. 2022. 279-341 pp. doi: 10.52305/GXME8274.
- Min J, Koenig J, Nashiro K, et al. Resting heart rate variabi–lity is associated with neural adaptation when repeatedly exposed to emotional stimuli. Neuropsychologia. 2024 Apr 15;196:108819. doi: 10.1016/j.neuropsychologia.2024.108819.
- Moretta T, Messerotti Benvenuti S. Early indicators of vulnerability to depression: The role of rumination and heart rate variability. J Affect Disord. 2022 Sep 1;312:217-224. doi: 10.1016/j.jad.2022.06.049.
- Porges SW. Polyvagal Theory: A science of safety. Front Integr Neurosci. 2022 May 10;16:871227. doi: 10.3389/fnint.2022.871227.
- Hu JR, Abdullah A, Nanna MG, Soufer R. The Brain-heart axis: neuroinflammatory interactions in cardiovascular disease. Curr Cardiol Rep. 2023 Dec;25(12):1745-1758. doi: 10.1007/s11886-023-01990-8.
- Brandt T, Huppert D. Brain beats heart: A cross-cultural reflection. Brain. 2021 Jul 28;144(6):1617-1620. doi: 10.1093/brain/awab080.
- Coll MP, Hobson H, Bird G, Murphy J. Systematic review and meta-analysis of the relationship between the heartbeat-evoked potential and interoception. Neurosci Biobehav Rev. 2021 Mar;122:190-200. doi: 10.1016/j.neubiorev.2020.12.012.
- Lambiase PD, Garfinkel SN, Taggart P. Psychological stress, the central nervous system and arrhythmias. QJM. 2023 Dec 27;116(12):977-982. doi: 10.1093/qjmed/hcad144.
- Fang S, Zhang W. Heart-Brain Axis: A narrative review of the interaction between depression and arrhythmia. Biomedicines. 2024 Aug 1;12(8):1719. doi: 10.3390/biomedicines12081719.
- Engelen T, Solcà M, Tallon-Baudry C. Interoceptive rhythms in the brain. Nat Neurosci. 2023 Oct;26(10):1670-1684. doi: 10.1038/s41593-023-01425-1.
- Lamotte G, Shouman K, Benarroch EE. Stress and central autonomic network. Auton Neurosci. 2021 Nov;235:102870. doi: 10.1016/j.autneu.2021.102870.
- Akil H, Nestler EJ. The neurobiology of stress: Vulnerability, resilience, and major depression. Proc Natl Acad Sci USA. 2023 Dec 5;120(49):e2312662120. doi: 10.1073/pnas.2312662120.
- Sun Y, Lu Y, Saredy J, et al. ROS systems are a new integra–ted network for sensing homeostasis and alarming stresses in organelle metabolic processes. Redox Biol. 2020 Oct;37:101696. doi: 10.1016/j.redox.2020.101696.
- Forte G, Casagrande M. The intricate brain-heart connection: The relationship between heart rate variability and cognitive functio–ning. Neuroscience. 2025 Jan 26;565:369-376. doi: 10.1016/j.neuroscience.2024.12.004.
- Sun S, Yu H, Yu R, Wang S. Functional connectivity between the amygdala and prefrontal cortex underlies processing of emotion ambiguity. Transl Psychiatry. 2023 Oct 28;13(1):334. doi: 10.1038/s41398-023-02625-w.
- Olivieri F, Biscetti L, Pimpini L, Pelliccioni G, Sabbatinelli J, Giunta S. Heart rate variability and autonomic nervous system imbalance: potential biomarkers and detectable hallmarks of aging and inflammaging. Ageing Res Rev. 2024 Nov;101:102521. doi: 10.1016/j.arr.2024.102521.
- Sobolewska-Nowak J, Wachowska K, Nowak A, et al. Exploring the heart-mind connection: unraveling the shared pathways between depression and cardiovascular diseases. Biomedicines. 2023 Jul 5;11(7):1903. doi: 10.3390/biomedicines11071903.
- Westhoff-Bleck M, Lemke LH, Bleck JS, Bleck AC, Bauer–sachs J, Kahl KG. Depression associated with reduced heart rate variabi–lity predicts outcome in adult congenital heart disease. J Clin Med. 2021 Apr 7;10(8):1554. doi: 10.3390/jcm10081554.
- Hadaya J, Dajani AH, Cha S, et al. Vagal nerve stimulation reduces ventricular arrhythmias and mitigates adverse neural cardiac remodeling post-myocardial infarction. JACC Basic Transl Sci. 2023 Jun 7;8(9):1100-1118. doi: 10.1016/j.jacbts.2023.03.025.
- Siepmann M, Weidner K, Petrowski K, Siepmann T. Heart rate variability: A measure of cardiovascular health and possible the–rapeutic target in dysautonomic mental and neurological disorders. Appl Psychophysiol Biofeedback. 2022 Dec;47(4):273-287. doi: 10.1007/s10484-022-09572-0.
- Tomasi J, Zai CC, Pouget JG, Tiwari AK, Kennedy JL. Heart rate variability: Evaluating a potential biomarker of anxiety disorders. Psychophysiology. 2024 Feb;61(2):e14481. doi: 10.1111/psyp.14481.
- Cui L, Li S, Wang S, Wu X, et al. Major depressive disorder: hypothesis, mechanism, prevention and treatment. Signal Transduct Target Ther. 2024 Feb 9;9(1):30. doi: 10.1038/s41392-024-01738-y.
- Karki P, Shahi PV, Sapkota KP, Bhandari R, Adhikari N, Shrestha B. Depressive symptoms and associated factors among persons with physical disabilities in disability care homes of Kathmandu district, Nepal: A mixed method study. PLOS Glob Public Health. 2023 Jan 12;3(1):e0001461. doi: 10.1371/journal.pgph.0001461.
- Watkins ER, Roberts H. Reflecting on rumination: consequences, causes, mechanisms and treatment of rumination. Behav Res Ther. 2020 Apr;127:103573. doi: 10.1016/j.brat.2020.103573.
- Zareian B, Wilson J, LeMoult J. Cognitive control and ruminative responses to stress: understanding the different facets of cognitive control. Front Psychol. 2021 May 7;12:660062. doi: 10.3389/fpsyg.2021.660062.
- Patel A, Daros AR, Irwin SH, et al. Associations between rumination, depression, and distress tolerance during CBT treatment for depression in a tertiary care setting. J Affect Disord. 2023 Oct 15;339:74-81. doi: 10.1016/j.jad.2023.06.063.
- Hartmann R, Schmidt FM, Sander C, Hegerl U. Heart rate variability as indicator of clinical state in depression. Front Psychiatry. 2019 Jan 17;9:735. doi: 10.3389/fpsyt.2018.00735.
- Ramesh A, Nayak T, Beestrum M, Quer G, Pandit JA. Heart rate variability in psychiatric disorders: A systematic review. Neuropsychiatr Dis Treat. 2023 Oct 20;19:2217-2239. doi: 10.2147/NDT.S429592.
- Carnevali L, Thayer JF, Brosschot JF, Ottaviani C. Heart rate variability mediates the link between rumination and depressive symptoms: A longitudinal study. Int J Psychophysiol. 2018 Sep;131:131-138. doi: 10.1016/j.ijpsycho.2017.11.002.
- Dell’Acqua C, Dal Bo’ E, Messerotti Benvenuti S, Palomba D. Reduced heart rate variability is associated with vulnerability to depression. J. Affect. Disorders Rep. 2020;1:100006. doi: 10.1016/j.jadr.2020.100006.
- Solanki JD, Hirani CN, Vohra AS, Panjwani SJ, Senta VM, Rudani DK. A comparative cross-sectional study of cardiac autonomic status by five minute heart rate variability among type 2 diabetics, hypertensives and normotensive-nondiabetics. Niger Med J. 2023 Jul 2;64(3):373-381. doi: 10.60787/NMJ-64-3-204.
- Ortiz-Guzmán JE, Mollà-Casanova S, Serra-Añó P, et al. Short-term heart rate variability in metabolic syndrome: A systematic review and meta-analysis. J Clin Med. 2023 Sep 19;12(18):6051. doi: 10.3390/jcm12186051.
- Besson C, Baggish AL, Monteventi P, et al. Assessing the clini–cal reliability of short-term heart rate variability: insights from controlled dual-environment and dual-position measurements. Sci Rep. 2025;15:5611. doi: 10.1038/s41598-025-89892-3.
- Azulay N, Olsen RB, Nielsen CS, et al. Reduced heart rate variability is related to the number of metabolic syndrome components and manifest diabetes in the sixth Tromsø study 2007-2008. Sci Rep. 2022 Jul 14;12(1):11998. doi: 10.1038/s41598-022-15824-0.
- Castiglioni P, Faini A, Nys A, et al. Heart rate variability for the early detection of cardiac autonomic dysfunction in type 1 diabetes. Front Physiol. 2022 Jun 30;13:937701. doi: 10.3389/fphys.2022.937701.
- Valensi P. Autonomic nervous system activity changes in patients with hypertension and overweight: role and therapeutic implications. Cardiovasc Diabetol. 2021 Aug 19;20(1):170. doi: 10.1186/s12933-021-01356-w.
- Immanuel S, Teferra MN, Baumert M, Bidargaddi N. Heart rate variability for evaluating psychological stress changes in healthy adults: A scoping review. Neuropsychobiology. 2023;82(4):187-202. doi: 10.1159/000530376.
- Serhiyenko V, Sehin V, Pankiv V, Serhiyenko A. Post-trauma–tic stress disorder, dyssomnias, and metabolic syndrome. Mìžnarodnij endokrinologìčnij žurnal. 2024;20(1):58-67. doi: 10.22141/2224-0721.20.1.2024.1359.
- Xu L, Zhai X, Shi D, Zhang Y. Depression and coronary heart disease: mechanisms, interventions, and treatments. Front Psychiatry. 2024 Feb 9;15:1328048. doi: 10.3389/fpsyt.2024.1328048.
- Hadad R, Akobe SF, Weber P, et al. Parasympathetic tonus in type 2 diabetes and pre-diabetes and its clinical implications. Sci Rep. 2022;12:18020. doi: 10.1038/s41598-022-22675-2.
- Yugar LBT, Yugar-Toledo JC, Dinamarco N, et al. The role of heart rate variability (HRV) in different hypertensive syndromes. Diagnostics (Basel). 2023 Feb 19;13(4):785. doi: 10.3390/diagnostics13040785.
- Geraldes V, Laranjo S, Nunes C, Rocha I. Central autono–mic network regions and hypertension: unveiling sympathetic activation and genetic therapeutic perspectives. Biology (Basel). 2023 Aug 21;12(8):1153. doi: 10.3390/biology12081153.
- Leo DG, Ozdemir H, Lane DA, Lip GYH, Keller SS, Proiet–ti R. At the heart of the matter: how mental stress and negative emotions affect atrial fibrillation. Front Cardiovasc Med. 2023 Jun 20;10:1171647. doi: 10.3389/fcvm.2023.1171647.
- Xavier A, Noble S, Joseph J, Ghosh A, Issac TG. Heart rate and its variability from short-term ECG recordings as potential biomarkers for detecting mild cognitive impairment. Am J Alzheimers Dis Other Demen. 2024 Jan-Dec;39:15333175241309527. doi: 10.1177/15333175241309527.
- Jin H, Li M, Jeong E, Castro-Martinez F, Zuker CS. A body-brain circuit that regulates body inflammatory responses. Nature. 2024 Jun;630(8017):695-703. doi: 10.1038/s41586-024-07469-y.
- Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020 Jul 22;107(2):234-256. doi: 10.1016/j.neuron.2020.06.002.
- Serhiyenko VA, Sehin VB, Serhiyenko LM, Serhiyenko AA. Post-traumatic stress disorder, metabolic syndrome, and the autono–mic nervous system. Endokrynologia. 2023 Dec;28(4):377-392. doi: 10.31793/1680-1466.2023.28-4.377.
- Petruso F, Giff AE, Milano BA, De Rossi MM, Saccaro LF. Inflammation and emotion regulation: a narrative review of evidence and mechanisms in emotion dysregulation disorders. Neuronal Signal. 2023 Nov 15;7(4):NS20220077. doi: 10.1042/NS20220077.
- Syed SA, Beurel E, Loewenstein DA, et al. Defective inflammatory pathways in never-treated depressed patients are associated with poor treatment response. Neuron. 2018 Sep 5;99(5):914-924.e3. doi: 10.1016/j.neuron.2018.08.001.
- Iob E, Kirschbaum C, Steptoe A. Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: the role of cognitive-affective and somatic symptoms. Mol Psychiatry. 2020 May;25(5):1130-1140. doi: 10.1038/s41380-019-0501-6.
- Zhang Y, Mei H, Xiao H, et al. Association between neutrophil-lymphocyte ratio and perinatal depressive symptoms among Chinese women. J Psychosom Res. 2023 Mar;166:111101. doi: 10.1016/j.jpsychores.2022.111101.
- Serhiyenko VA, Sehin VB, Serhiyenko LM, Serhiyenko AA. Post-traumatic stress disorder, metabolic syndrome, and chronic low-grade inflammation: A narrative review. Problemi Endocrinnoi Patologii. 2024 Mar 14;81(1):77-83. doi: 10.21856/j-PEP.2024.1.10.
- Andreadi A, Muscoli S, Tajmir R, et al. Recent pharmacological options in type 2 diabetes and synergic mechanism in cardiovascular di–sease. Int J Mol Sci. 2023 Jan 13;24(2):1646. doi: 10.3390/ijms24021646.
- Zhao X, An X, Yang C, Sun W, Ji H, Lian F. The crucial role and mechanism of insulin resistance in metabolic disease. Front Endocrinol (Lausanne). 2023 Mar 28;14:1149239. doi: 10.3389/fendo.2023.1149239.
- Serhiyenko VA, Serhiyenko AA, Segin VB, Serhiyenko LM. Association of arterial stiffness, N-terminal pro-brain natriuretic peptide, insulin resistance, and left ventricular diastolic dysfunction with diabetic cardiac autonomic neuropathy. Vessel Plus. 2022;6:4515. doi: 10.20517/2574-1209.2021.83.
- Bakkar NZ, Dwaib HS, Fares S, Eid AH, Al-Dhaheri Y, El-Yazbi AF. Cardiac autonomic neuropathy: A progressive consequence of chronic low-grade inflammation in type 2 diabetes and related metabolic disorders. Int J Mol Sci. 2020 Nov 27;21(23):9005. doi: 10.3390/ijms21239005.
- Galicia-Garcia U, Benito-Vicente A, Jebari S, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020 Aug 30;21(17):6275. doi: 10.3390/ijms21176275.
- Zong Y, Li H, Liao P, et al. Mitochondrial dysfunction: mechanisms and advances in therapy. Signal Transduct Target Ther. 2024 May 15;9(1):124. doi: 10.1038/s41392-024-01839-8.
- Guo Q, Jin Y, Chen X, et al. NF-κB in biology and targeted therapy: new insights and translational implications. Signal Transduct Target Ther. 2024 Mar 4;9(1):53. doi: 10.1038/s41392-024-01757-9.
- Sudo SZ, Montagnoli TL, Rocha BS, Santos AD, de Sá MPL, Zapata-Sudo G. Diabetes-induced cardiac autonomic neuropathy: Impact on heart function and prognosis. Biomedicines. 2022 Dec 15;10(12):3258. doi: 10.3390/biomedicines10123258.
- Serhiyenko VA, Serhiyenko LM, Sehin VB, Serhiyenko AA. Pathophysiological and clinical aspects of the circadian rhythm of arterial stiffness in diabetes mellitus: A minireview. Endocr Regul. 2022 Oct 20;56(4):284-294. doi: 10.2478/enr-2022-0031.
- Hansen CS, Vistisen D, Jørgensen ME, et al. Adiponectin, biomarkers of inflammation and changes in cardiac autonomic function: Whitehall II study. Cardiovasc Diabetol. 2017 Dec 1;16(1):153. doi: 10.1186/s12933-017-0634-3.
- Herranz N, Gil J. Mechanisms and functions of cellular senescence. J Clin Invest. 2018;128:1238-1246. doi: 10.1172/JCI95148.
- Sayed N, Huang Y, Nguyen K, et al. An infammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nat Aging. 2021;1:598-615. doi: 10.1038/s43587-021-00082-y.
- Serrano-López J, Martín-Antonio B. Infammaging, an imba–lanced immune response that needs to be restored for cancer prevention and treatment in the elderly. Cells. 2021;10:2562. doi: 10.3390/cells10102562.
- Matacchione G, Perugini J, Di Mercurio E, et al. Senescent macrophages in the human adipose tissue as a source of infammaging. Geroscience. 2022;44:1941-1960. doi: 10.1007/s11357-022-00536-0.
- Williams DP, Koenig J, Carnevali L, et al. Heart rate variabi–lity and inflammation: A meta-analysis of human studies. Brain Behav Immun. 2019 Aug;80:219-226. doi: 10.1016/j.bbi.2019.03.009.
- Li G, Zhang L, Liu M. Meta-analysis on inflammation and autonomic nervous system of coronary heart disease combined with depression. BMJ Open. 2024 Mar 7;14(3):e079980. doi: 10.1136/bmjopen-2023-079980.
- Singh S, Singh Sh, Shukla N, Shukla A. Association of heart rate variability and C-reactive protein in patients with depression. J Family Med Prim Care. 2024 Jan;13(1):191-198. doi: 10.4103/jfmpc.jfmpc_1020_23.
- Al-Rashed F, Sindhu S, Al Madhoun A, et al. Elevated res–ting heart rate as a predictor of inflammation and cardiovascular risk in healthy obese individuals. Sci Rep. 2021 Jul 6;11(1):13883. doi: 10.1038/s41598-021-93449-5.
- Qian Z, Yang H, Li H, et al. The cholinergic anti-inflammatory pathway attenuates the development of atherosclerosis in Apoe-/- mice through modulating macrophage functions. Biomedicines. 2021 Sep 3;9(9):1150. doi: 10.3390/biomedicines9091150.
- Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020 Jun 15;877:173090. doi: 10.1016/j.ejphar.2020.173090.
- Giuliani A, Giudetti AM, Vergara D, et al. Senescent endothelial cells sustain their senescence-associated secretory phenotype (SASP) through enhanced fatty acid oxidation. Antioxidants (Basel). 2023 Nov 2;12(11):1956. doi: 10.3390/antiox12111956.
- Schiweck C, Sausmekat S, Zhao T, Jacobsen L, Reif A, Edwin Thanarajah S. No consistent evidence for the anti-inflammatory effect of vagus nerve stimulation in humans: A systematic review and meta-analysis. Brain Behav Immun. 2024 Feb;116:237-258. doi: 10.1016/j.bbi.2023.12.008.
- Sharan P, Vellapandian C. Hypothalamic-pituitary-adrenal (HPA) axis: Unveiling the potential mechanisms involved in stress-induced Alzheimer’s disease and depression. Cureus. 2024 Aug 23;16(8):e67595. doi: 10.7759/cureus.67595.
- Lian L, Zhang Y, Liu L, et al. Neuroinflammation in ischemic stroke: focus on microRNA-mediated polarization of microglia. Front Mol Neurosci. 2021;13:612439. doi: 10.3389/fnmol.2020.612439.
- Barthel MC, Fricke K, Muehlhan M, Vogel S, Alexander N. Habituation of the biological response to repeated psychosocial stress: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2025 Feb;169:105996. doi: 10.1016/j.neubiorev.2024.105996.
- Ge F, Yuan M, Li Y, Zhang W. Posttraumatic stress disorder and alterations in resting heart rate variability: A systematic review and meta-analysis. Psychiatry Investig. 2020 Jan;17(1):9-20. doi: 10.30773/pi.2019.0112.
- Li B, Guo S, Xu H, et al. Abnormal circadian rhythm of heart rate variability and their association with symptoms in patients with major depressive disorder. J Affect Disord. 2024 Oct 1;362:14-23. doi: 10.1016/j.jad.2024.06.102.
- Im B, Keum J, Kim T, Lee K-i, Koo K-i. Utilizing real-time heart rate variability during psychological intervention program for complex post-traumatic stress disorder: A case study. Appl Sci. 2024; 14(1):4. doi: 10.3390/app14010004.
- Lee Y, Walsh RJ, Fong MWM, Sykora M, Doering MM, Wong AWK. Heart rate variability as a biomarker of functional outcomes in persons with acquired brain injury: Systematic review and meta-analysis. Neurosci Biobehav Rev. 2021 Dec;131:737-754. doi: 10.1016/j.neubiorev.2021.10.004.
- Qiu Q, Song W, Zhou X, et al. Heart rate variability is associated with cerebral small vessel disease in patients with diabetes. Front Neurol. 2022 Nov 10;13:989064. doi: 10.3389/fneur.2022.989064.
- Jiang Y, Yabluchanskiy A, Deng J, Amil FA, Po SS, Dasari TW. The role of age-associated autonomic dysfunction in inflammation and endothelial dysfunction. Geroscience. 2022 Dec;44(6):2655-2670. doi: 10.1007/s11357-022-00616-1.
- Al Jowf GI, Ahmed ZT, Reijnders RA, de Nijs L, Eijssen LMT. To predict, prevent, and manage post-traumatic stress disorder (PTSD): A review of pathophysiology, treatment, and biomarkers. Int J Mol Sci. 2023 Mar 9;24(6):5238. doi: 10.3390/ijms24065238.
- Zhao M, Guan L, Wang Y. The association of autonomic nervous system function with ischemic stroke, and treatment strategies. Front Neurol. 2020 Jan 22;10:1411. doi: 10.3389/fneur.2019.01411.